| 001 | | STUDYID | Study Identifier | Char | Populate as "MYCSG" | |
| 002 | | DOMAIN | Domain Abbreviation | Char | Populate as "PP" | |
| 003 | | USUBJID | Unique Subject Identifier | Char | Populate by concatenating "MYCSG-" to the value of subject as a prefix. | |
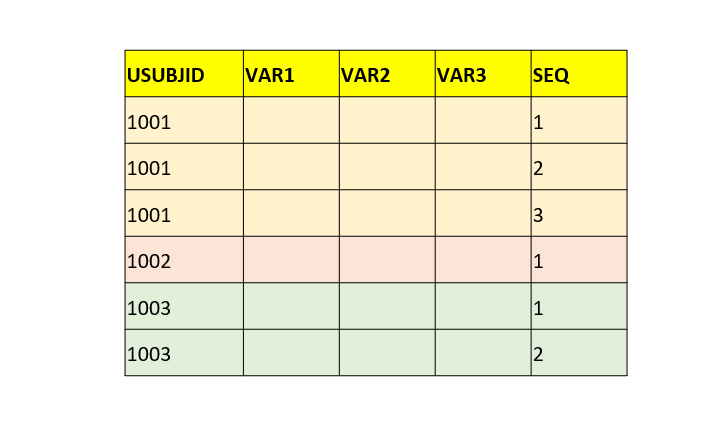
| 004 | | PPSEQ | Sequence Number | Num | Sort the records by USUBJID, PPCAT, PPTESTCD, PPGRPID and set to 1 on the first record for each subject, and increment by 1 for each subsequent record within the subject.
 | |
| 005 | | PPGRPID | Group ID | Char | | Populate with the value from GRPID variable. |
| 006 | | PPTESTCD | Parameter Short Name | Char | | Populate with the first word from PARAM variable where '(' is the delimiter. |
| 007 | | PPTEST | Parameter Name | Char | | Set to "Time of CMAX" when PPTESTCD="TMAX".
Set to "Max Conc" when PPTESTCD="CMAX".
Set to "AUC All" when PPTESTCD="AUCALL".
Set to "Half-Life Lambda z" when PPTESTCD="LAMZHL".
Set to "Vz Obs" when PPTESTCD="VZO".
|
| 008 | | PPCAT | Parameter Category | Char | | Populate with the second word from GRPID variable where '_' is the delimiter. |
| 009 | | PPORRES | Result or Finding in Original Units | Char | | Populate with the value from RESULT variable. |
| 010 | | PPORRESU | Original Units | Char | | Populate with the second word from PARAM variable where '(', ')' are the delimiters. |
| 011 | | PPSTRESC | Character Result/Finding in Std Format | Char | Populate with the value from PPORRES variable | |
| 012 | | PPSTRESN | Numeric Result/Finding in Standard Units | Num | Convert PPSTRESC to numeric value where applicable. | |
| 013 | | PPSTRESU | Standard Units | Char | Populate with the value from PPORRESU variable | |
| 014 | | PPSPEC | Specimen Material Type | Char | | Populate with the value from SPEC variable. |
| 015 | | PPRFTDTC | Date/Time of Reference Point | Char | | Populate using PCON dataset. Merge PPARAM and PCON datasets at subject and GRPID variables level and fetch DOSING_TIME from PCON as PPRFTDTC.
Notes: 1) Use only first word from GRPID variable of PPARAM dataset.
2) Multiple records exist in PCON for each SUBJECT and GRPID - first record can be used as DOSING_TIME is expected to be same on all records of a GRPID. |
